Adolescent & Young Adult Sarcomas
(P 11) LX-101, A NOVEL, CLINICAL STAGE, PAYLOAD-BEARING TARGETED THERAPY DIRECTED TO THE INSULIN-LIKE GROWTH FACTOR RECEPTOR, HAS POTENT PRECLINICAL ANTI-TUMOR ACTIVITY AGAINST PEDIATRIC SARCOMAS
Location: The Liffey, Level 1

Matthew Hoberman, BSE
VP Operations
Lirum Therapeutics
New York, New York, United States
Author(s)
Objective: LX-101, a next-generation, targeted therapy directed to the insulin-like growth factor 1 receptor (IGF-1R), consists of a proprietary IGF-1 variant coupled to a cytotoxic methotrexate (MTX) payload. LX-101 was previously evaluated in Phase 1 trials of adult patients with advanced, pretreated cancers, where it was well-tolerated and demonstrated single agent activity. Neither a dose limiting toxicity, nor a maximum tolerated dose were reached, leaving potential room for additional dose escalation and schedule optimization. Several aggressive pediatric sarcomas of unmet need have genetic alterations affecting the IGF-1R pathway and/or have high IGF-1R expression that correlates with poor outcomes, including Ewing’s sarcoma, rhabdomyosarcoma, osteosarcoma, and others. Prior attempts at inhibiting the IGF-1R with non-payload bearing naked monoclonal antibodies or small molecule tyrosine kinase inhibitors produced a range of clinical outcomes in Ewing’s and other related sarcomas, including some partial and complete responses. None of these agents, however, were ultimately approved in an oncology setting, potentially due to suboptimal potency that allowed for redundant signaling pathways and other escape mechanisms. Here, we investigated the anti-tumor activity of the payload-bearing agent LX-101 against pediatric sarcoma cell lines in vitro.
Methods: Cell lines were incubated with LX-101 (~1.6 - 2500nM). Cell viability was assessed by CellTiter-Glo after 4 days. IC50s were calculated using GraphPad PRISM software.
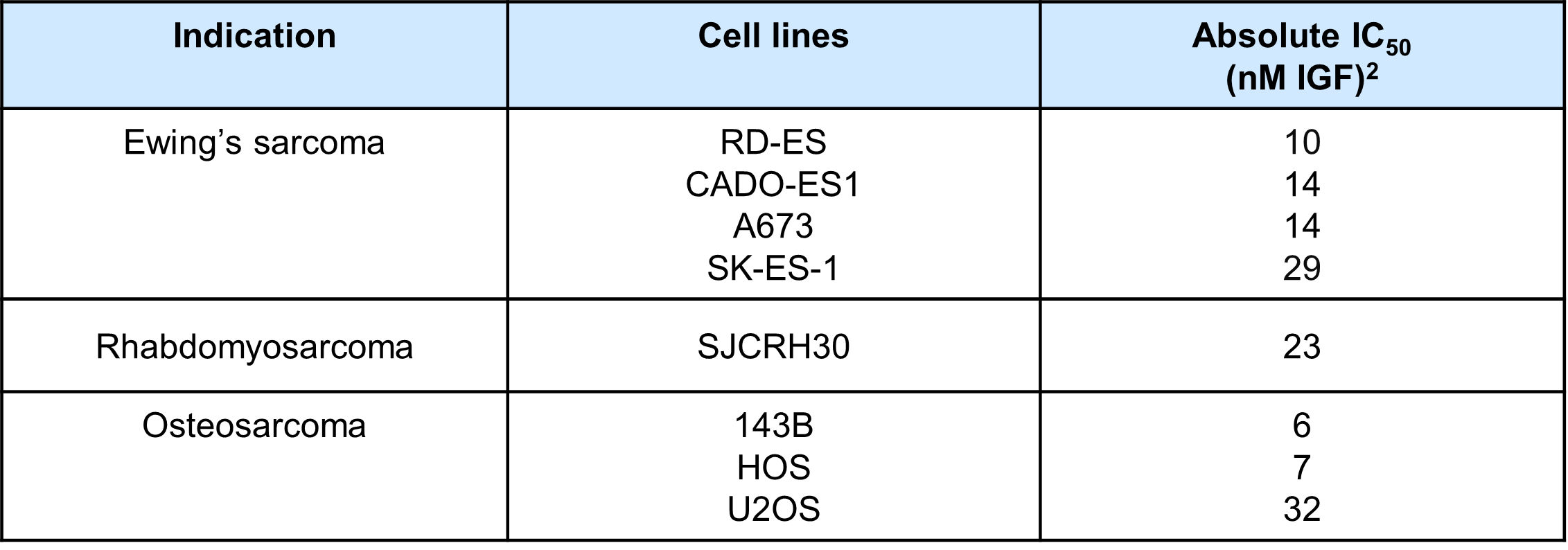
Results: The results are summarized in Table 1. All Ewing’s sarcoma cell lines tested were sensitive to LX-101, including EWSR1-FLI1 gene fusion-positive cell lines RD-ES (IC50 = 10 nM), A-673 (IC50 = 14 nM), and SK-ES-1 (IC50 = 29 nM) and EWSR1-ERG gene fusion-positive cell line CADO-ES1 (IC50 = 14 nM). LX-101 was also potent against PAX3-FOXO1 gene fusion positive alveolar rhabdomyosarcoma (SJCRH30, IC50 = 23 nM) and osteosarcoma (143B, IC50 = 6 nM; HOS, IC50 = 7 nM; U2OS, IC50 = 32 nM).
Conclusion: These results demonstrate that LX-101 has potent preclinical anti-tumor activity against pediatric sarcoma cell lines with well-established ties to the IGF-1R pathway, including with different oncogenic gene fusions. These data further support the clinical development of LX-101 in IGF-1R-related pediatric cancers. A clinical trial is planned.
Methods: Cell lines were incubated with LX-101 (~1.6 - 2500nM). Cell viability was assessed by CellTiter-Glo after 4 days. IC50s were calculated using GraphPad PRISM software.
Results: The results are summarized in Table 1. All Ewing’s sarcoma cell lines tested were sensitive to LX-101, including EWSR1-FLI1 gene fusion-positive cell lines RD-ES (IC50 = 10 nM), A-673 (IC50 = 14 nM), and SK-ES-1 (IC50 = 29 nM) and EWSR1-ERG gene fusion-positive cell line CADO-ES1 (IC50 = 14 nM). LX-101 was also potent against PAX3-FOXO1 gene fusion positive alveolar rhabdomyosarcoma (SJCRH30, IC50 = 23 nM) and osteosarcoma (143B, IC50 = 6 nM; HOS, IC50 = 7 nM; U2OS, IC50 = 32 nM).
Conclusion: These results demonstrate that LX-101 has potent preclinical anti-tumor activity against pediatric sarcoma cell lines with well-established ties to the IGF-1R pathway, including with different oncogenic gene fusions. These data further support the clinical development of LX-101 in IGF-1R-related pediatric cancers. A clinical trial is planned.


